
Am I suitable to take part?
We are inviting people aged 18 years or over who have active rheumatoid arthritis and who are about to start treatment with a DMARD medicine, or changing their current DMARD medicine to consider taking part in the LEADER study. You will not be able to take part if you have uncontrolled diabetes, are pregnant, have received steroid treatment within the past 3 months or have been diagnosed with fibromyalgia or chronic widespread pain in the last six months.
What would my involvement be?
LEADER is a randomised study, which means that the steroid treatment a patient receives is randomly chosen by a computer. Those that agree to take part will be selected at random to receive one of the following four treatments: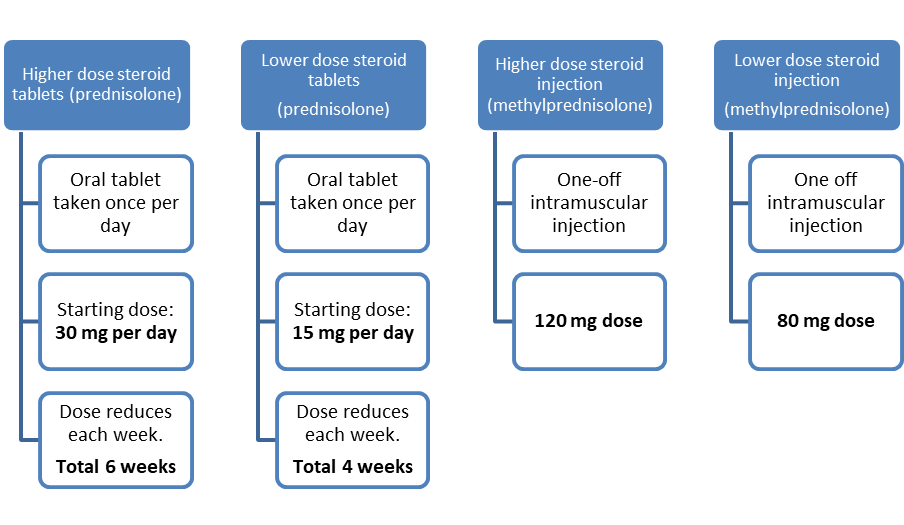
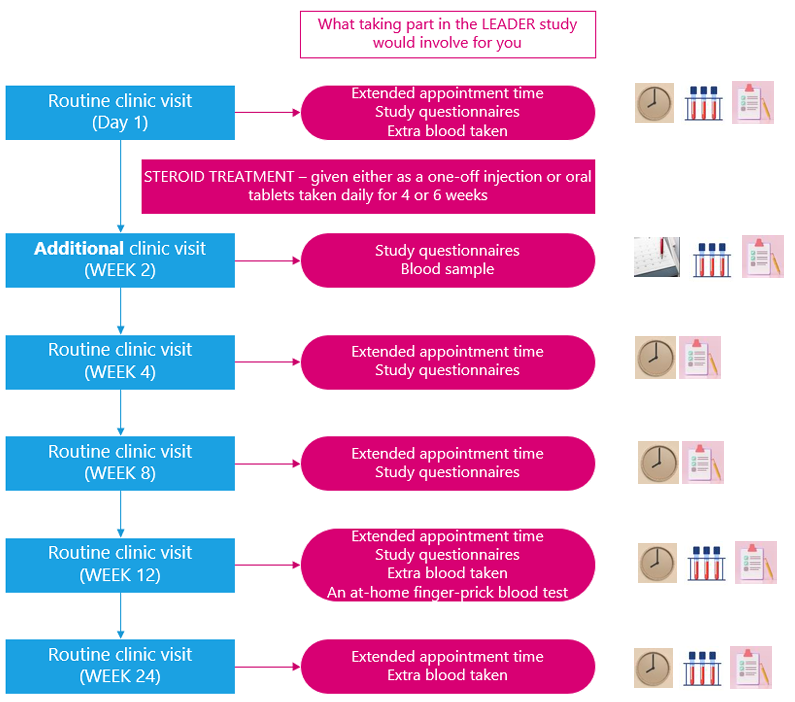
This diagram shows when there would be anything asked of you for the LEADER Study:
If you have any questions about the LEADER trial, please contact the Leader study team at the University of Oxford via the following email address: leader@ndorms.ox.ac.uk
You can also reach the Oxford Clinical Trials Research Unit on the following number: 0808 281 5345






